Berkeley Lab Researchers Evaluate Tin Nanocrystals for Li-ion Battery Electrodes
The energy density in lithium-ion (Li-ion) batteries enables them to store and provide considerable energy in a small package. Powering devices from pacemakers to smart phones, these small, lightweight, rechargeable batteries have become a ubiquitous part of modern electronics. Using materials such as tin and silicon give Li-ion even a higher energy density—enough for a battery to power an electric vehicle—but the electrochemical reactions with those metals produce extremely high volume changes, which lead to mechanical stress and failure.
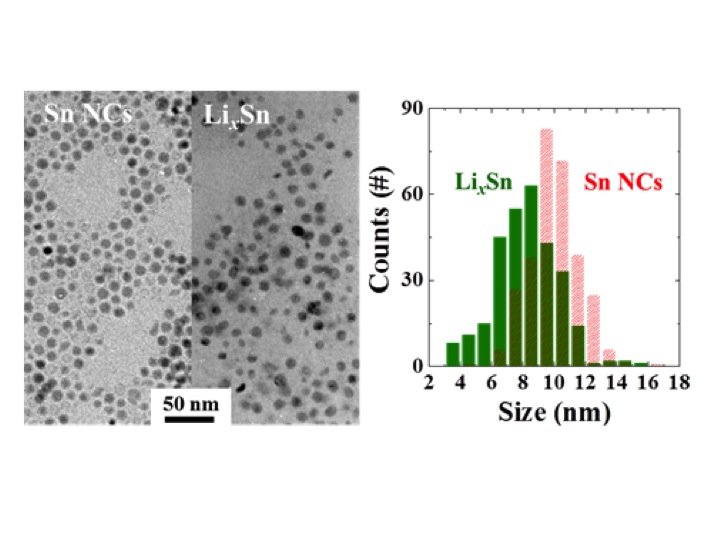
Many battery researchers have suggested that by using nanocrystals, this mechanical damage may be avoided, so researchers at Lawrence Berkeley National Laboratory’s (Berkeley Lab’s) Environmental Energy Technologies Division (EETD) and Molecular Foundry tested that theory with tin nanocrystals. They studied mechanical cracking on homogenous 10 nanometer (nm) tin crystals from electrochemical cycling with lithium. The nanomaterial exhibited better cyclability than commercially available particles; however, an ex situ transmission electron microscopy (TEM) examination of the sample showed significant damage after the first lithiation, proving that a size reduction at a 10 nm scale does not prevent the material from cracking.
These results could refocus the research in this area. Because this work suggests that particle damage may be inevitable when using tin nanocrystals for Li-ion battery electrodes, future research may be better directed toward the creation of self-healing structures or strategies to make the surface of the particles unreactive with the electrolyte. The researchers noted, however, that this work may not apply to silicon, which has already been shown to resist cracking during expansion.
This project was conducted by EETD researchers Linping Xu, Chunjoong Kim, Alpesh K. Shukla, and Jordi Cabana and Molecular Foundry researchers Angang Dong, Tracy M. Mattox and Delia J. Milliron. It was funded by the U.S. Department of Energy (DOE) under the Batteries for Advanced Transportation Technologies (BATT) Program.