Chemical Analysis at the Nanometer Scale—Berkeley Lab Breakthrough Uses Laser, Near Field Optics
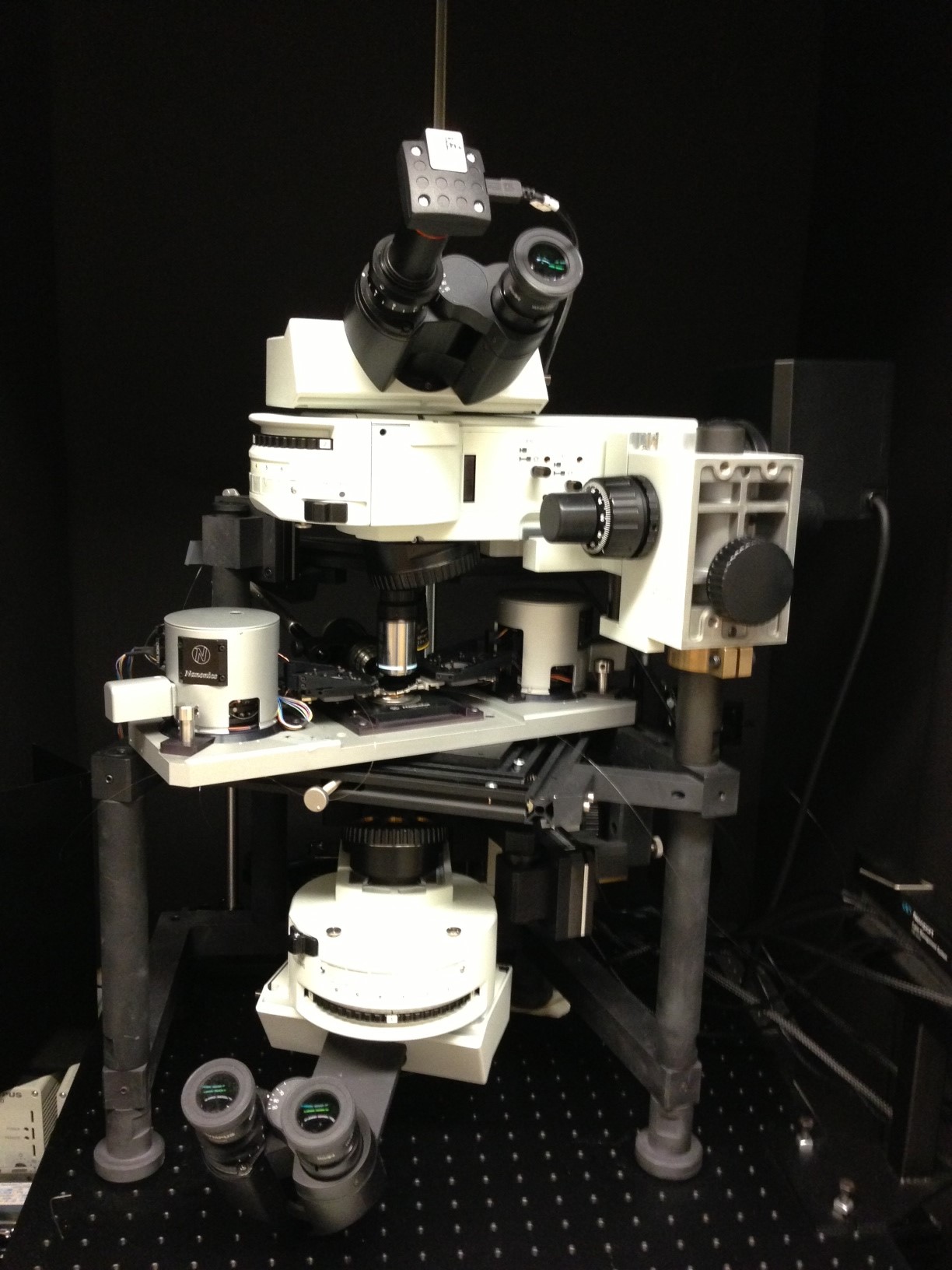
For 30 years or so, researchers in the Laser Spectroscopy and Applied Materials Group at Lawrence Berkeley National Laboratory (Berkeley Lab) have been studying laser beams.
Berkeley Lab's Rick Russo—a pioneer in the field—has been working with a technology called Laser Induced Breakdown Spectroscopy (LIBS), refining and expanding the technology for many applications. Famous for its use on Mars by the rover Curiosity in 2012, LIBS uses a laser beam to vaporize a small sample of material (called laser ablation), then uses a telescope to view the vapor, or plasma, and then uses a spectrometer to analyze the light emitted from the plasma.
Since every material has a unique spectral signature, thanks to its chemical components, this "light signature" is like a fingerprint, allowing researchers to identify components in the material.
Out of this research at Berkeley Lab was born another technology called LAMIS—or Laser Ablation Molecular Isotopic Spectrometry. Both LIBS and LAMIS use laser ablation to analyze samples in just a few seconds. But where LIBS only measures the optical emission spectra of atoms and ions, LAMIS measures the emission spectra of molecules and molecular ions. This enables LAMIS to identify the specific isotopes of a chemical element within the plasma plume. Russo won an R&D 100 award in 2012 for his work using the LAMIS technology.
Five years ago, a new researcher at Berkeley Lab, Vasileia Zormpa, joined the Energy Storage and Distributed Research Department to take their chemical imaging work even further. Based on the original LIBS technology, and refined through LAMIS, Zormpa is the first person to advance this capability to use laser ablation to analyze very small samples—on the nanometer spatial scale.
Focusing the Beam
Taking smaller samples requires more tightly focused laser beams, the greatest challenge of this research. Basic laws of physics limit spatial resolution: light cannot be focused to dimensions lower than roughly half the wavelength of the laser beam used, which for conventional laser systems is on the order of a few hundred nanometers. To circumvent these limits, Zormpa and her team are incorporating a more sophisticated concept called "near-field optics" and combining it with ultra-fast lasers (or femtosecond lasers), which pulse at a duration of a millionth of a billionth of a second.
Near-field optics allows them to focus the femtosecond laser beams to the microscopic size of a few tens to a few hundred nanometers. Moving the laser beam very close to the sample material—5 to 10 nanometers from the sample—allows them to focus the beam very tightly, achieving high spatial resolution, which means that the laser ablates a much smaller amount of material during each pulse.
As a comparison, an average human hair measures about 75,000 nanometers, a virus measures 30-50 nanometers, and DNA measures about 2.5 nanometers.
"My job is to get the beam focused," Zormpa said. "When the lasers interact with the surface of the material, it will vaporize a portion of the material. We are interested in how small a sample size we can take, the best spatial resolution we can achieve," she said.
But a smaller sample size, with less vaporized plasma, creates other challenges to overcome. As the material is vaporized, it generates a tiny spark of light—a specific spectrum that is captured and analyzed. If the amount of material is very small, less light is available. Zormpa's research looks at the most efficient way to balance the goals of focusing the beam while still being able to read and analyze the chemical information needed.
"We have been able to still get chemical information with beam sizes less than 500 nanometers, which is a world record in laser ablation optical chemical imaging," Zormpa said. "The amount of material we vaporized was 200 attograms—a billionth of a billionth of a gram," she said.
Why So Small?
In the past, laser ablation technologies have been used to test long-distance for explosives, test toys and paint for lead contamination, and assess contamination at hazardous waste sites—without the chemicals to dissolve samples or vacuum chambers that other sampling techniques require.
In addition, LAMIS represents what may be the only practical means of determining the geochronology of samples on Mars or other celestial bodies in the Solar System. Strontium isotope ratios have been a focus in the field of medicine for both treatment and diagnostic purposes. Measuring these ratios can provide valuable information about atmospheric chemistry. They also can be used to trace the origins and movements of early humans. Perhaps the most immediate and important application of LAMIS will be in nuclear forensics aimed at non-proliferation and terrorism.
But this new near-field technology is used in other applications that require careful and precise—and very small—samples. Over the past few years, Zormpa and her group have been applying these ultra-focused (near- and far-field) femtosecond beam technologies to analyzing solar and battery research.
For example, working with Robert Kostecki's battery group at Berkeley Lab, Zormpa is using femtosecond laser ablation technology to help improve batteries for electric vehicles. In their research, they are studying a thin, interfacial layer on the batteries that is very important for the performance and safety of the system. Because the layer is very thin, only about 50 nanometers thick, using lasers with lower depth resolution (sampling thick slices of material at a time) doesn't work.
"Let's say you have a small particle, only a few nanometers wide, next to another particle, one of silicon and one of aluminum," she said. "If you use a larger beam size, you vaporize both materials, and you don't have a clear picture of where each individual particle was," she said.
The main strength of the near-field ablation technology has to do with true three-dimensional imaging. Using the ultra-focused lasers, Zormpa can determine the composition of the material, how elements are distributed in the layer, and test for impurities.
"We have been successful because we were able remove thin slices with laser pulses, about seven nanometers at a time," Zormpa said. "Each layer is analyzed so that we have a three-dimensional 'picture' of the elements and composition of the sample," she said.
Zormpa and her group are also working with solar cells, developing the technology to test and analyze whether materials have impurities—which decreases the efficiency of the electricity output. And they are in the process of developing a portable tool that can provide the three-dimensional image of the constituents of materials like solar cells and batteries to help with research and development.
"Solar manufacturers want to know the concentration of impurities or dopants in the cell and their exact location," Zormpa said. "If they know, they can figure out how to manage and improve the efficiency."
Looking to the Future
While current research has allowed Zormpa and her team to use near-field optics to ablate smaller and more precise masses in their samples (excitation), the detection of the material's chemical signature was based on traditional far-field optics.
"In our current work we are developing a new and unique system that allows us to both excite AND detect in the near-field," Zormpa said. "We expect that this approach will help us improve the spatial resolution and limits of detection significantly."